Guess who you might see in the next few weeks in your neighborhood, Brood X stragglers!
Last May in 2021 cicada lovers exulted in the arrival of billions of periodical cicadas in the eastern United States. By mid-June as the party wound down, they bemoaned the fact that in most of the DMV these strange and magnificent creatures would not return until the spring of 2038. But guess what, last week on an early morning walk on a trail in Columbia, MD, I was surprised and delighted to encounter a freshly molted male pharaoh periodical cicada, Magicicada septendecim, scaling a gnarly ancient red maple tree. Nearby beneath the same tree, a second male dodged running shoes and bicycle tires roaring down the asphalt. My sightings mirrored reports of cicada sightings in more than a dozen states in the eastern half of the US. These off-cycle sightings of a few periodical cicadas are part of the ongoing mystery surrounding one of Nature’s most magical creatures. Before local cicadaphiles get their hopes up too high and cicadaphobes start packing to leave town, please know that this is not the full-blown cicadapalooza of 2021. Brood X cicadas will be seen throughout the land but at densities many orders of magnitude less than those seen last year.
Against the background calls of Canadian geese and mallard ducks, a male Brood X cicada scales an ancient maple tree in the early morning light. Watch as this lonesome bachelor avoids entanglement in a spider’s web. Instinct drives his quest to find a mate. Little does he know that his chances of passing along his genes to the next generation are between slim and none.

In several states in the eastern half of the US, shed skins appearing on plants in your landscape during the next several weeks are likely those of straggling Brood X cicadas.
Cicada experts call sightings of a few cicadas in “off” years, cicada “stragglers.” Stragglers are periodical cicadas that emerge in years prior to or after the year that massive numbers of their broodmates are expected to emerge. Often, 17-year cicada stragglers emerge four years prior to their expected emergence date; however, it is possible for periodical cicadas to emerge between 8 years earlier or 4 years later than expected. Based on historical data, researchers can associate stragglers with their massive parent brood. The map accompanying this episode provides scientifically vetted accounts of actual sightings of periodical cicadas in our region this spring. This wonderful event has entomologists eager to add new information to our knowledge of these inimitable creatures. Experts believe that part of the straggling phenomenon is genetic while environmental factors, such as the quality of the host tree immature cicadas dine on while underground, contribute to the appearance of stragglers. Sadly, densities of stragglers in an area rarely achieve a quorum great enough to overwhelm hungry predators and other foes, and their unfortunate off-cycle appearance leads to oblivion for their progeny.
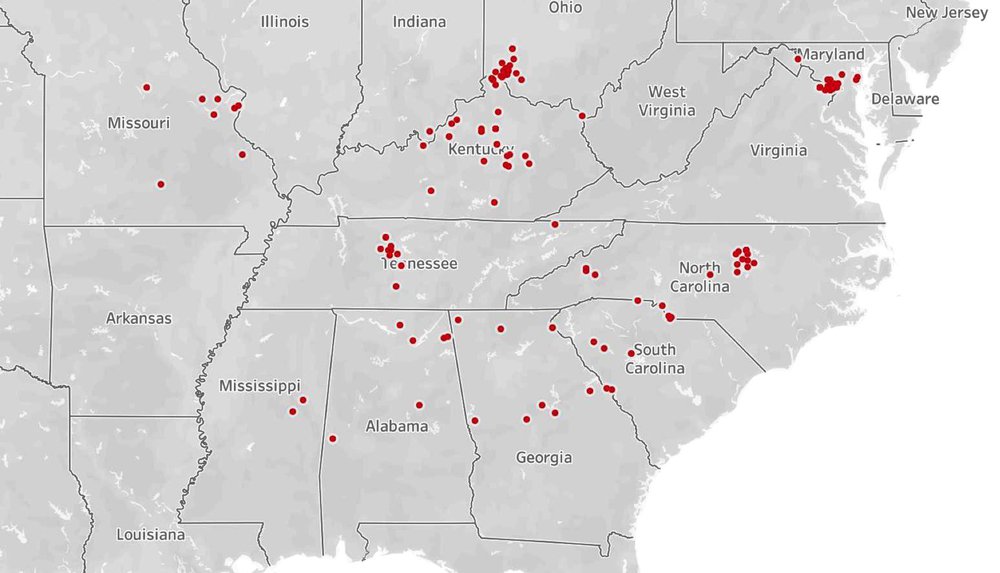
This recent map compiled from data sent to iNaturalist and Cicada Safari apps shows locations where Brood X cicada stragglers are likely to be seen this spring. Credit: Gene Kritsky, Mount St. Joseph University
Cicadaphiles, don’t despair, as this spring provides one more chance to enjoy cicadas and to help scientists learn more about these creatures. You can participate in the highly successful community science project that resulted in hundreds of thousands of data points last year by joining the Cicada Safari. To be part of the action, go to the app store on your cellular phone and download the Cicada Safari app. It is free and very easy to use. Download, register, and start snapping pictures of cicadas. Easy as pie. Cicada geniuses will vet your images and add them to a growing data base designed to demystify the seasonal phenology and distribution of these charismatic creatures. On this Memorial Day Holiday and over the next several weeks as you enjoy parades, cookouts, and adventures in the great outdoors, keep your cell phones handy, eyes open and ears on the ready, and snap some shots of straggling Brood X cicadas.
Acknowledgements
We thank Dr. Gene Kritsky of Mount St. Joseph University for providing the nice map of recent cicada sightings and for providing inspiration for this episode. To learn more about magical periodical cicadas, please visit the fabulous repository for all things cicada at Cicada Mania and search the archives at Bug of the Week for “cicada.” To read John Kelly’s take on tardy cicadas here in the DMV in the Washington Post, please click on this link: https://www.washingtonpost.com/dc-md-va/2022/05/22/cicadas-emerging-broodx/. The wonderful fact-filled review of cicada biology and ecology, “Advances in the Evolution and Ecology of 13- and 17-Year Periodical Cicadas” by Chris Simon, John R. Cooley, Richard Karban, and Teiji Sota was consulted for this episode.